Electrical energy associated with moving charges or electric current.
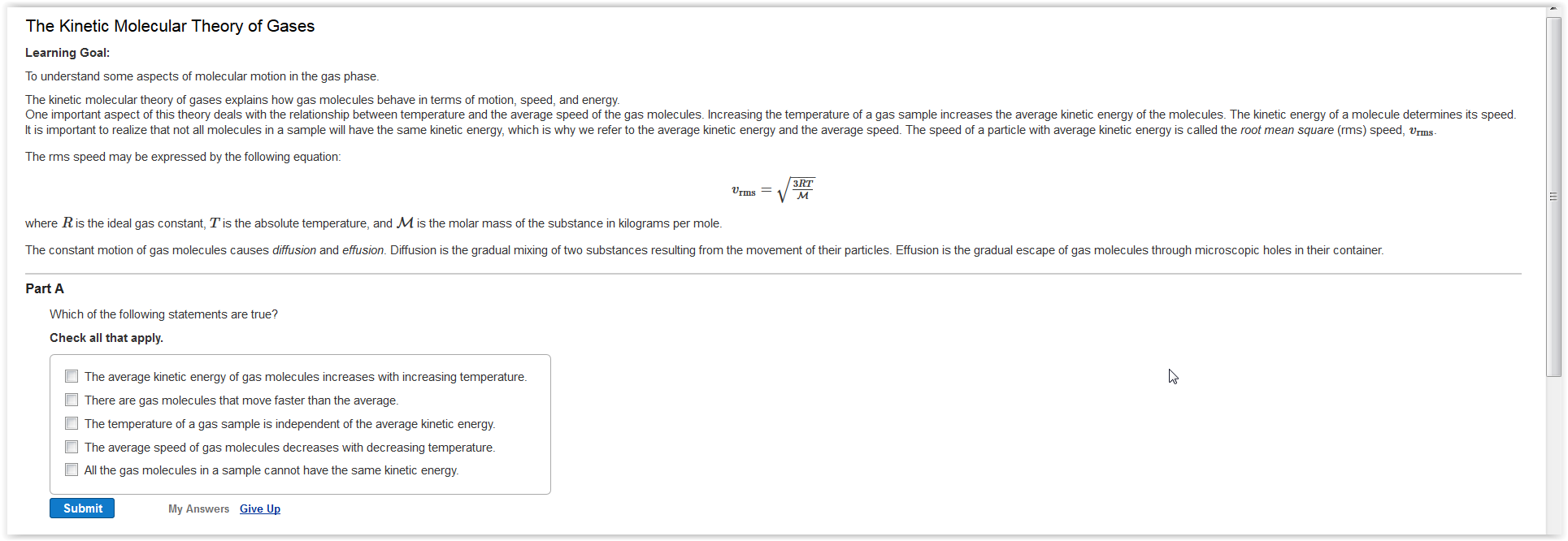
What is the average kinetic energy of moving particles called.
The kinetic energy of the particles in an object determines the object s temperature.
So by increasing the temperature of the system the particles move faster.
At any given temperature not all of the particles of a sample of matter have the same kinetic energy.
Particles in any matter are in constant motion.
Each particle has mass and size.
As the charges that cause the energy are moving electrical energy is a form of kinetic energy.
As stated in the kinetic molecular theory the temperature of a substance is related to the average kinetic energy of the particles of that substance when a substance is heated some of the absorbed energy is stored within the particles while some of the energy increases the motion of the particles.
Electrical energy is caused by moving electric charges called electrons the faster the charges move the more electrical energy they carry.
The higher the temperature the higher the average energy.
However a small number of particles have kinetic energies a great deal lower or.
Instead the particles display a wide range of kinetic energies.
Lightning batteries and even electric eels are examples of electrical energy in action.
Due to constant and random motion each particle has energy.
The temperature of the gas is related to the average energy in the kinetic energy stores of the gas particles.
Kinetic energy and temperature.
Kinetic energy form of energy that an object or a particle has by reason of its motion.
The energy of motion is known as kinetic energy.
Options in motion change direction moving either up forward down or backwards.
The kind of motion may be translation rotation about an axis vibration or any combination of motions.
Protons neutrons and electrons are the most known examples of these particles.
Most of the particles have a kinetic energy near the middle of the range.
The average kinetic energy of the particles in an object determines the object s.
The kinetic theory of gases is a historically significant but simple model of the thermodynamic behavior of gases with which many principal concepts of thermodynamics were established the model describes a gas as a large number of identical submicroscopic particles atoms or molecules all of which are in constant rapid random motion their size is assumed to be much smaller than the.
The average kinetic energy of the particles of a substance is determined by the temperature of the medium using the equation for an ideal gas.
Kinetic energy is a property of a moving object or particle and depends not only on its motion but also on its mass.
These particles have intertia and occupy physical space.
If the temperature is unknown then the average speed and mass of the particles are utilized to determine the average kinetic energy.