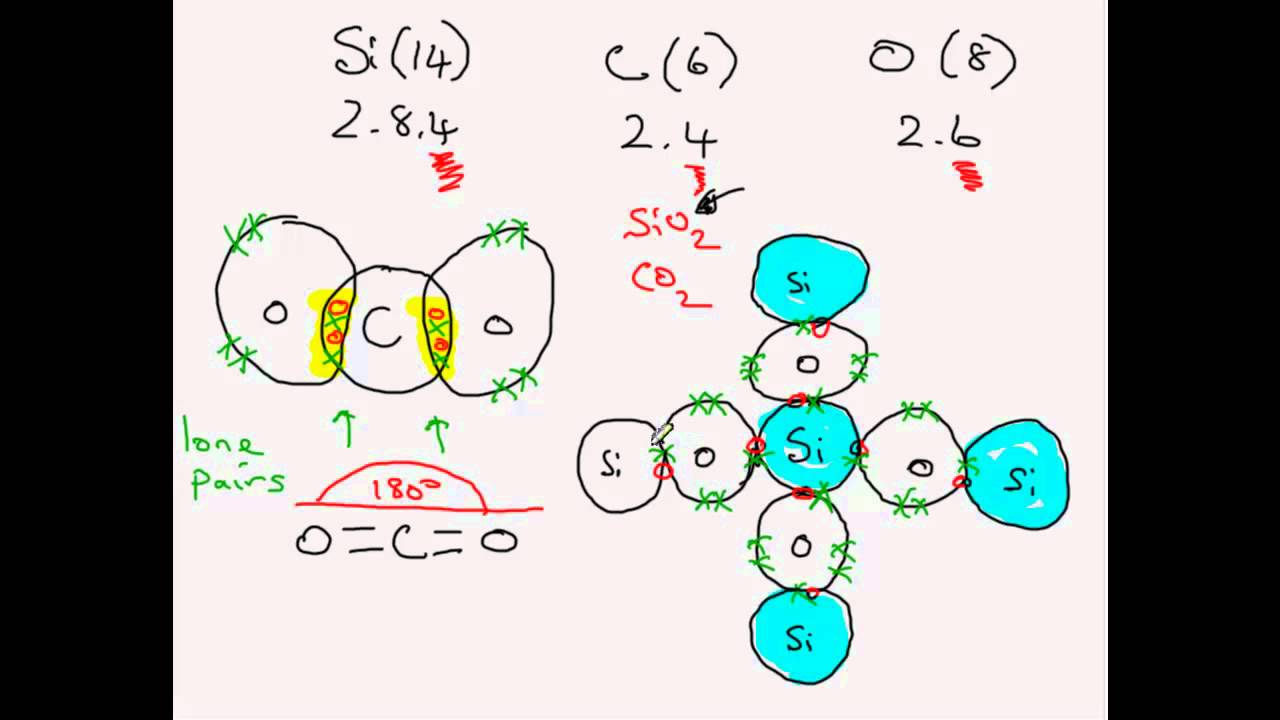
In case of sio2 each si atom is linked with o atom with single bond thus forming a network solid with high melting point 4 6k views.
Why is carbon dioxide a gas at room temperature and silicon dioxide a solid.
O c o each molecule is attracted to other molecules because of what is called van der waal s forces or london forces.
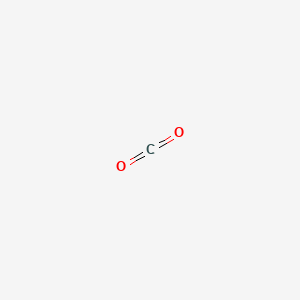
Carbon dioxide is a linear structure with two double bonds between carbon and oxygen.
It is a small molecule and non polar with only weak bonds between the molecules.
Carbon dioxide consists of molecules in which a single carbon atom is double bonded to two oxygen atoms.
In math co 2 math displayed formula.
The reason why carbon dioxide is a gas and silicon dioxide is a solid is because their chemical structures are different.
Thus co2 is a gas at room temperature.
Then at that temperature molecules of co2 are tightly bonded together to be in solid form.
Carbon dioxide is a gas at room temperature silicon iv oxide is a solid with a high.
At a very low temperatures like 80 degrees centigrade co2 can become a solid and it is called dry ice.
They have a lot of energy compared to molecules in a solid.
As co2 is.
Hence it is a gas.
Silicon dioxide is giant molecular.
Explain why silicon dioxide is a solid and carbon dioxide is a gas at room temperature.
This is at room temperature.
1 educator answer silicon iv oxide has a high melting point whereas carbon dioxide is a gas.
Although c and si are both group 4 elements c is much smaller than si and can form double bonds with two oxygen atoms whereas si is.
Explain this by comparing their particles and those forces between these particles.
Carbon dioxide is simple molecular.