The next most common gas is oxygen at 21.
Why is nitrogen an inert gas at room temperature.
Nitrogen compounds have a very long history ammonium chloride having been known to herodotus they were well known by the middle ages.
Nitrogen is present as n2 or dinitrogen.
The reason why these elements form gases at room temperature is that the diatomic molecules that they both form have relatively little attraction for eachother and therefore they move.
The triple bond is pretty strong.
The noble gases often do not react with many substances and were historically referred to as the inert gases.
An inert gas is a gas that does not undergo chemical reactions under a set of given conditions.
These undesirable chemical reactions are often oxidation and hydrolysis reactions with the oxygen and.
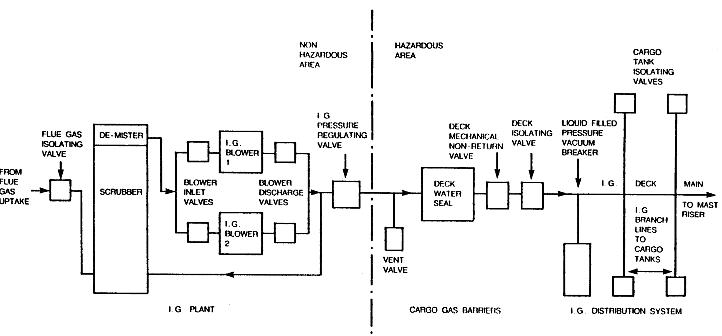
Inert gases are used generally to avoid unwanted chemical reactions degrading a sample.
Nitrogen has a low melting and boiling point and is a gas at room temperature.
The mixture of nitric and hydrochloric acids was known as aqua regia royal water celebrated for its ability to dissolve.
That is why nitrogen is inert in room temperature state the following 1 volume of gas at 0 degree kelvin2 degree celsius 3 gas equation4 ice point in absolute temperature 5 stp conditions 1.